Call Dr. Glicksman 732-974-2424
Get Up to Speed on the Popular Implant Material
1976
- Congress approved the Medical Device Amendments Act, which provided the FDA the authority to regulate devices. This gave the FDA the ability to go back and review all devices that had previously been “grandfathered” by the industry.
- In July 1976, the Plastic Surgery Device Advisory Panel classified implants as Class II devices. The FDA classifications for devices are based upon those that are legally marketed in the United States. The FDA determines the device classification by both the specific device’s intended use and any potential risk the device presents to the patient. The FDA classifies breast implants as Class II medical devices, and they must comply with special controls that include special labeling requirements, mandatory performance standards, postmarket surveillance, and FDA medical device specific guidance. Although the FDA established these new guidelines, they did not enact them.

1988
- It was not until 1988 that the FDA finally took on the task to classify breast implants, but instead of classifying them as Class II they classified them as Class III devices. Class III medical devices have the tightest controls of all medical devices. Class III medical devices are usually those that support or sustain human life, such as heart valves and cerebellum stimulators. Class III devices require strict pre-market approval (PMA) submission to the FDA before a device can be approved for use in the United States. In addition to the PMA requirements, the implant factories must also be inspected by the FDA to verify the manufacturing of the devices. This process can often take several years.
- The new classification of silicone breast implants as Class III medical devices in June of 1988 initiated the FDA review process, and manufacturers were set to the task for the first time to prove with compelling scientific data that their devices were safe and effective.
1990
- CBS airs “Face to Face” with Connie Chung, and the popular media encourages a surge of panic. Viewers across the United States are presented with a documentary with obvious deficiencies in evidence-based science on the reported complications associated with silicone gel breast implants. Women frantically call their plastic surgeons for information. There are even reports of women attempting to remove their own implants. The door is opened for hundreds of legal claims across the country against plastic surgeons, as well as the manufacturers. Large-sum settlements begin to be awarded to women with claims of “autoimmune” illnesses, without the support of peer-reviewed scientific data.
1991
- September: The FDA concludes that the manufacturer’s safety data does not prove that devices are safe—or harmful.
- November: The FDA holds a General and Plastic Surgery Devices Panel to review all safety data from the leading manufacturers of the time: Dow Corning, Mentor, McGhan, and Bioplasty. They concluded there that the manufacturers and their scientists provided insufficient safety data.
- December: Large settlements continue to be awarded by the courts to “victims” of silicone breast implants by Dow Corning and other manufacturers.
1992
- January: FDA Commissioner David Kessler calls for a voluntary moratorium on the distribution and sale of all breast implants containing silicone gel.
- April: The FDA lifts its absolute moratorium on silicone-filled breast implants and allows the continued use of silicone implants for women who have had, or will need, surgery for breast reconstruction, or if they have existing implants or complications. Furthermore, for the first time in the United States, women who are to receive silicone breast implants must be enrolled in FDA Adjunct Clinical trials to ensure the collection of prospective data and long-term follow-up.
- December: By the end of the year, only two manufacturers have elected to continue to manufacture silicone breast implants for import into the United States: McGhan Medical and Mentor.
1993-1994
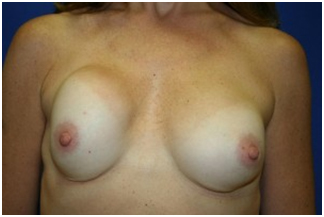
- Lawsuits are filed by thousands of women across the United States who claim damages due to their exposure to silicone breast implants. The single largest class action suit is against Dow Corning, which made its first silicone implant for Dr. Thomas Cronin in 1962. The company provided almost 30 percent of the silicone implants used in the United State by the 1980s, although breast implants accounted for less than 1 percent of its annual sales. Monetary amounts are awarded to women who are able to prove that their silicone breast implants are the cause of their ailments.
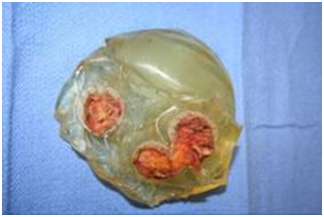
- Unfortunately, due to the insufficient scientific data on the relationship between silicone breast implants and systemic illnesses, there is very little “science” available in the courtrooms.
June 1994
- A Mayo Clinic study in the New England Journal of Medicine finds no increased risk of connective tissue disease or other disorders as result of silicone breast augmentation or reconstruction (N Engl J Med 1994; 330:1697-1702 June 16, 1994).
1995
- The American College of Rheumatology states that they do not see compelling evidence of any cause and effect relationship between silicone breast implants and connective tissue disease. Two large studies were reviewed (NEJM 33D: 1697-702, 1995) and (NEJM 332: 1666-70, 1995). They concluded that anecdotal evidence should no longer be used to support claims in the courts or by the FDA.
- December: More than 20 studies and abstracts from the United States conclude that there is no causal relationship between silicone breast implants and autoimmune illnesses.
1997
- The American Academy of Neurology concludes its review of the existing literature on silicone implants and concludes “existing research shows no link between silicone breast implants and neurological disorders.”
- The Journal of the National Cancer Institute publishes its review of clinical studies and concludes that silicone breast implants are not associated with an increased risk of breast cancer. A large number of epidemiological studies looking into any potential associations between silicone breast implants and breast cancer, as well as other cancers, provides good evidence that silicone breast implants do not result in a higher frequency of breast cancer (Berkel et al. , 1992, Brinton et al. , Bryant and Brasher, 1995, Deapen et al. , 1997, Friis et al. , 1997, Glasser et al. , 1989 Kern et al. , 1997).
- The International Journal of Cancer publishes its review of “Breast implants and cancer risk in Denmark” (Volume 71, Issue 6, pages 956–958, 11 June 1997)
1998
- The Journal of Neurology publishes a Scandinavian abstract that dismisses “silicone-induced neurologic disease.” In addition, a second study in the same journal titled “Breast Implant Risk of Neurologic Disease” concluded that there was no scientific evidence behind the speculation that breast implants cause any neurologic disease (Nyren et al., 1998).
- The Institute of Medicine (IOM) concludes that there is convincing evidence that infants breast-fed by mothers with silicone breast implants receive no higher silicon intake from breast milk than infants breast fed by mothers without implants. In addition, children are routinely fed Simethicone-containing drops for gastric colic, with no known adverse effects.
1999

- The Institute of Medicine completes and publishes its 400-page report on the “Safety of Silicone Breast Implants.” The comprehensive study integrated the findings of 13 independent experts from diverse multi-specialty backgrounds. They conclude that, although silicone gel implants will not last a lifetime and may lead to local complications such as gel bleed and capsular contracture, they do not produce any known systemic illnesses. This landmark publication turns the tide in the silicone gel controversy, introducing volumes of research and data on the safety and efficacy of silicone gel breast implants.
2000-2004
- Inamed Medical (Allergan), Silimed (Sientra), and Mentor all begin core studies in the United States with newer-generation shaped silicone gel implants. This newer generation of shaped, textured devices is designed with a wide portfolio of shapes and sizes. There exists varying degrees of cohesiveness between the manufacturers’ implants, as well as differences in the characteristics of the elastomer shells. Each manufacturer selects a group of principal investigators, and several manufacturers provide intensive training to these plastic surgeons in the use of their newest shaped devices.
2003, 2005, 2011

- The FDA Center for Devices and Radiological Health Advisory panels convenes in Gaithersburg, Virginia. Expert testimony is reviewed from the manufacturers, medical experts, and patients. Opposition groups also present at hearings, providing anecdotal case reports.
- Capitol Hill Briefing on the Safety of Silicone Breast Implants: Members of Congress and their staff attend a briefing to discuss the safety and efficacy of silicone gel breast implants.
October 17, 2006
- The FDA announces the release of Inamed’s and Mentor’s silicone gel-filled breast implants back into the U.S. market, with new restrictions. The silicone devices may be used for breast augmentation in women over the age of 22 and for all revisions and reconstructions. Both manufacturers must comply with 10-year post market approval studies.
November 2011
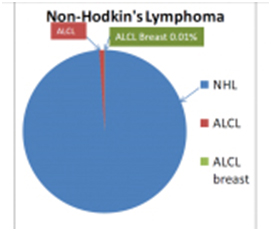
- The FDA releases a press statement concerning a rare possible association between silicone gel breast implants and a rare form of lymphoma called Anaplastic Large Cell Lymphoma (ALCL). Worldwide studies are initiated to further document this extremely rare condition. The FDA concludes, however, that silicone gel implants remain a safe and effective medical device.
January 2012

- Poly Implant Prothese (PIP) was involved in considerable worldwide controversy when the French manufacturer of saline and silicone gel breast implants was banned from further sale of their devices. It was discovered that they had been using a less expensive non-medical grade silicone as the filler for their gel implants. In 2012, they filed for bankruptcy. These implants were never imported into the United States (See Blog on PIP breast implants and PSC: PIP implants).
March 9, 2012

- Sientra-Silamed receives FDA approval for their shaped and round silicone gel implants in the United States with restrictions similar to those required by Mentor and Allergan.
February 20, 2013
The FDA approves the Allergan Style 410 Highly cohesive gel breast implant. Restrictions are similar to those outlined in 2006, with the following additional conditions:
- Continue to follow, for an additional five years, approximately 3,500 women in the Continued Access Study
- Conduct 10-year study of more than 2,000 women
- Conduct five case control studies to evaluate the possible association between Natrelle 410 implants, as well as other silicone breast implants, and five rare diseases: rare connective tissue disease, neurological diseases, brain cancer, cervical-vulvar cancer, and lymphoma
- Evaluate women’s perceptions of the patient labeling
- Analyze the Natrelle 410 implants that are removed from patients and returned to the manufacturer
- Allergan includes information on the rare possible association of BIA-ALCL identified globally in some women with implants. This is the first mention of a possible “very small but increased risk of developing ALCL in the fluid of scar capsule adjacent to the implant. Because of the small numbers of cases worldwide, there was no consensus on etiology or treatment.
November 2014
Ideal saline breast implant is approved offering patients a newer saline implant option. The device was approved after 2 years of core data and the ten-year data has not yet been published
March 25-26, 2019

The General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee holds hearings to discuss the risks and benefits of breast implants indicated for augmentation and reconstruction. The topics included BIA-ALCL; systemic symptoms reported by patients receiving breast implants; the use of breast implant registries for breast implant surveillance; the use of MRI as a screening for silent rupture; the use of surgical mesh in breast procedures; the use of real-world data and patient reported outcomes in regulatory decision making; and proposed revisions to the informed consent process in breast implant surgery.
Get The Discussion Going With Dr. Glicksman
Learn more about Dr. Caroline Glicksman, a plastic surgeon in New Jersey, Call us to schedule a consultation today.
Dr. Glicksman In The Media
Dr. Glicksman is one of the founding members of the Plastic Surgery Channel and is a frequent contributor, serving on their Medical Advisory Board. Frequently asked to contribute to the health section of magazines like Allure, she also contributes educational content to many courses, textbooks, and peer-reviewed journals, including filming at WebMD studios in New York City.
View a selection of videos ranging from informative interviews about cosmetic and reconstructive procedures to real patients sharing their success stories. Tour her New Jersey practice, view Vectra® 3D Imaging simulations of results, check out the latest advances in surgical and nonsurgical techniques, and more.
Visit Our Video Gallery And Get To Know Dr. Glicksman